Strengthening Partnerships: Mochida Pharmaceuticals Visits Shannon HQ to discuss 2025 Collaborative Projects
Strengthening Partnerships: Mochida Pharmaceuticals Visits Shannon HQ to discuss 2025 Collaborative Projects The Mochida Pharmaceuticals leadership, quality and regulatory teams visited our headquarters in Shannon, Ireland this month, to discuss collaborative development projects for 2025 and beyond. We are pleased to share that the future is bright, with projects planned for trauma, tendon & ligament, as well as cavernous nerve. Pictured below is our Managing Partner, Paul Burke and Chief Science Officer, Gerard Insley presenting Hitoshi Mizuno, Head of Biomaterials business Division at Mochida Pharmaceuticals Ltd, with a plaque to mark the successful launch of ReFeel Nerve Repair Solution ‘Le Cheile’. #mochidapharamceuticals #ReFeelnerverepairsolution #acceleratingmedicalinnovation #partners

Another Successful Year at #OTA
Our team are celebrating another successful year at the Orthopaedic Trauma Association meeting, which was held in Montreal, QC last week. Our exciting pipeline of new medical innovations, including VAPS (a cell delivering #osteconductive scaffold), our Minimally Invasive Ankle Fixation Device, as well as our structural Bio-adhesive OsStic®, attracted huge levels of interest on our booth. Recently launched Nerve Repair Solution ‘ReFeel®’ was also a significant point of interest among attendees. In relation to our Education Pathway, the third instalment of the ‘Strengthening the Bond’ series was also well attended, with our panel of surgeon advisors discussing ‘Modern Biomaterial Solutions’ as well as facilitating a hands-on demonstrations of our ‘Breakthrough Biomaterial’ OsStic®.* Thank you to all those who stopped by the booth to speak to our team, and/or attended our symposium. We will see you all next year in Phoenix! #OTA2024 #acceleratingmedicalinnovation #PBCBiomed #biomaterials #orthopaedic #biomaterials #breakthrough *OsStic® is currently in development and not available for human use.

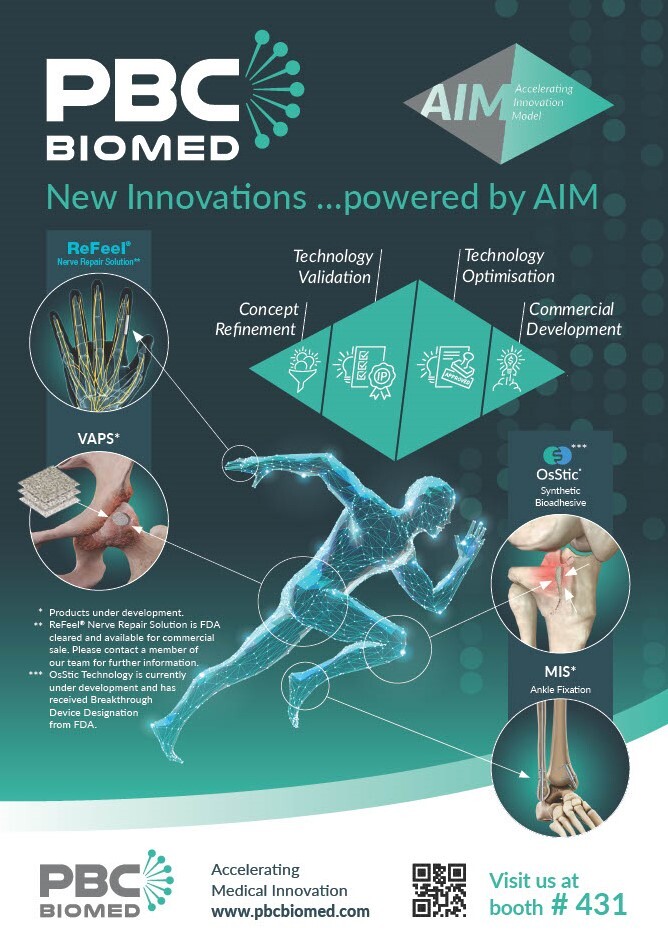
Accelerating new Medical Innovations: OTA Showcase
PBC Biomed are ready for action at the Orthopaedic Trauma Association meeting in Montreal! Stop by booth #431 to meet our team and learn more about our new #innovations, powered by our Accelerating Innovation Model ‘AIM’. #acceleratingmedicalinnovation #biomaterials #newinnovations
PBC Biomed OTA Symposium – Strengthening the Bond: Modern Biomaterials for Fracture Fixation and Bone Regeneration.
We are delighted to announce that we will be continuing our ‘Strengthening the Bond’ education series at the Orthopaedic Trauma Association meeting in Montreal this week. Strengthening the Bond: Modern Biomaterials for Fracture Fixation and Bone Regeneration. Our global panel of surgeon advisors will discuss current hashtag#Biomaterials and their challenges, as well as plans for the first human implantation of OsStic®, a hashtag#Breakthrough Bio-Adhesive bone void filler technology. Speaker panel is as below: – Peter Giannoudis – John Tracy Watson – Hassan Mir, MD, MBA – Thomas A. (Toney) Russell MD The session will take place on Friday 23rd October at 12:45pm in room 514ABC. See below for further information – we look forward to seeing you there! #OTA2024 #strengtheningthebond #industrylunchseries #pbcbiomed #acceleratingmedicalinnovation #biomimeticinnovations

DTIF Project Consortium Progress Meeting
It was fantastic to welcome the Enterprise Ireland #DTIF consortium team to our Shannon Headquarters at the start of September for a project progress meeting. Thank you to Nicholas Dunne and the DCU postgraduate team, the Dolmen Design & Innovation team, as well as Jackie FitzGerald of Enterprise Ireland for making the trip! #DTIF #disruptivetechnology #OsStic #development #researchanddevelopment #enterpriseireland #acceleratingmedicalinnovation

Launch of ReFeel® Nerve Repair Solution at ASSH 2024
PBC Biomed are delighted to announce the launch of our client Mochida Pharmaceutical Co., Ltd. innovative new nerve repair product – ReFeel® Nerve Repair Solution. ReFeel® is #biocompatible in nature and uses a proprietary alginate and PGA formula to create a bridge for #nerve #regeneration. The freeze-dried alginate sheet suppresses the migration and proliferation of cells that make up granulation tissue; providing a protected environment which facilitates the natural healing process of the nerve. The PBC Biomed Design & Development, Clinical, Regulatory and Commercial teams have been working alongside Mochida Pharmaceutical Co., Ltd. for the last four years; preparing this technology for commercial release. ReFeel® will officially be launched at the American Society for Surgery of the Hand (ASSH) conference in Minneapolis this week. Visit booth #210 for more information on this exciting new technology! Caution: Prescription use only. Federal law (USA) restricts this device to sale by or on the order of a physician. #acceleraratingmedicalinnovation #ASSH24

Launch of New PBC Biomed ‘What we Do’ Video
We are delighted to introduce our new company video, created by Christian Gyalus at Éalú Media. This video provides a fantastic insight into our mission, to enhance patient wellbeing by accelerating medical innovation; as well as the work that we do to achieve this mission. Huge thank you to Christian for making our vision a reality, and to the team for their support and contribution. We are thrilled with the end result! #pbcbiomed #acceleraratingmedicalinnovation #mission

ESTROT 2024
PBC Biomed are delighted to sponsor and confirm attendance at the 8th Annual European Society of Tissue Regeneration in Orthopaedics and Traumatology (ESTROT) meeting in Helsinki, Finland next week. This year’s event will focus on: – Biomaterials: tissue regeneration, infection & treatment– Bone & Cartilage: tissue reconstruction & regeneration– Traumatology: surgical techniques, host reactions– Plastic Surgery: soft tissue regeneration– Tissue Regeneration in Children We look forward to three days of stimulating discussion and learning, as well as an opportunity to catch up with European thought-leaders. Nähdään siellä! #ESTROT #trauma #regenerativemedicine #pbcbiomed #orthopaedics #trauma

DTIF-funded project, OsStic® technology awarded #BreakthroughDevice designation by the FDA.
Shannon, Ireland, February 12th 2024: Irish medical device company Biomimetic Innovations Ltd, an affiliate of PBC Biomed, announced ‘Breakthrough Device’ designation from the Food and Drug Administration FDA in January, for the development of a groundbreaking new bio-adhesive bone void filler technology called OsStic®. PBC Biomed leads a consortium for this development project, which is comprised of Biodesign Europe and SFI Research Centre for Advanced Manufacturing (I-Form) at Dublin City University, and Dolmen Design & Innovation, a Dublin-based product design company. The project (2021 – 2024) is funded under the Disruptive Technologies Innovation Fund (DTIF), which is a €500 million fund established under the National Development Plan (NDP) in 2018 and led by the Department of Enterprise, Trade and Employment, and administered by Enterprise Ireland. The aim of the fund is to drive collaboration between Ireland’s world-class research base and industry, as well as facilitating enterprises to compete directly for funding in support of the development and adoption of these technologies. Welcoming the announcement, Dr Imelda Lambkin, Disruptive Technologies and Innovation Department Manager, Enterprise Ireland commented; “This announcement is a great success for PBC Biomed and is testament to the capability and talent, vibrancy and international competitiveness of the Irish innovation and research system. In line with Enterprise Ireland’s strategy, this success will support the company to build on their existing capabilities, scale and create jobs. “The collaboration between Dublin City University, Dolmen Design & Innovation, Biomimetic Innovations, and PBC Biomed exemplifies the power of interdisciplinary efforts in research and development. The ‘Breakthrough Device’ designation by the FDA validates the promising trajectory of OsStic®, showcasing Ireland’s capability to lead in cutting-edge medical technologies. This achievement not only highlights the success of the project but also emphasizes the importance of sustained investment in disruptive technologies to foster innovation and elevate Ireland’s global standing in the medical device industry.” Professor Nicholas Dunne, Founding Executive Director of Biodesign Europe, Dublin City University and Funded Investigator in the SFI Research Centre for Advanced Manufacturing and SFI Centre for Advanced Materials and BioEngineering Research. “This announcement marks a major milestone in the evolution of calcium-based biomaterials. The proposed indication will be unique and for the first time will address all surgeon needs when dealing with complex fractures and allow for faster recovery times and greatly improved results for patients.” Gerard Insley, Chief Science Officer BMI. Learn more about DTIF here: https://lnkd.in/dHQ3_2vF About BMI Biomimetic Innovations Ltd, is an affiliate of PBC Biomed; a medical device company involved in design, development and manufacturing headquartered in Shannon, Ireland and with offices in Memphis, Tennessee and Chamonix, France.
European Society for Biomaterials 2022
European Society for Biomaterials Conference 2022 Event Name: ESB 2022 (European Society for Biomaterials 2022) Dates: September 5th – 8th 2022 Location: Bordeaux, France. Visit Us At: Booth 11

