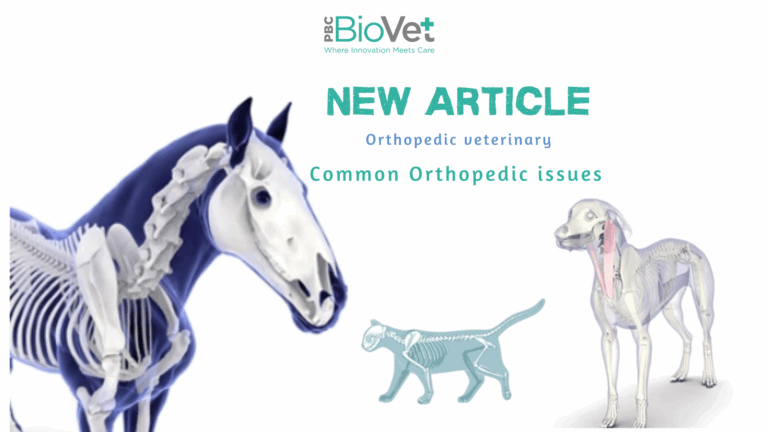
Common Orthopedic Issues in Veterinary Medicine
White Paper: Common Orthopedic Issues in Veterinary Medicine Introduction Orthopedic disorders are among the most prevalent health concerns in veterinary medicine, affecting companion animals, equine patients, and livestock. These conditions range from congenital deformities to acquired injuries and degenerative diseases, significantly impacting an animal’s mobility and quality of life. This white paper explores the most common orthopedic issues seen in veterinary practice, their causes, diagnostic approaches, and treatment options. 1. Fractures Causes and Risk Factors: Fractures occur due to trauma (e.g., vehicle accidents, falls) or underlying bone weaknesses such as metabolic bone disease or neoplasia. Types of Fractures: Diagnosis and Treatment: 2. Osteoarthritis (Degenerative Joint Disease) Causes and Risk Factors: Osteoarthritis (OA) is a progressive, degenerative disease resulting from joint wear and tear, trauma, or developmental abnormalities. Large breeds, overweight animals, and aging patients are at higher risk. Symptoms: Diagnosis and Treatment: 3. Hip Dysplasia Causes and Risk Factors: A genetic condition primarily affecting large dog breeds, hip dysplasia results from improper hip joint development, leading to instability and eventual arthritis. Symptoms: Diagnosis and Treatment: 4. Cruciate Ligament Rupture (ACL Injury) Causes and Risk Factors: Rupture of the cranial cruciate ligament (CCL) in dogs is a common cause of hind limb lameness, often resulting from obesity, genetic predisposition, or high-impact activities. Symptoms: Diagnosis and Treatment: 5. Luxating Patella Causes and Risk Factors: A common orthopedic issue in small dog breeds, patellar luxation occurs when the kneecap shifts out of its normal position due to congenital malformations or trauma. Symptoms: Diagnosis and Treatment: 6. Elbow and Shoulder Dysplasia Causes and Risk Factors: Developmental abnormalities in the elbow and shoulder joints, often hereditary, lead to joint instability and degeneration. Symptoms: Diagnosis and Treatment: 7. Intervertebral Disc Disease (IVDD) Causes and Risk Factors: IVDD occurs when spinal discs degenerate or herniate, compressing the spinal cord. It is common in chondrodystrophic breeds (e.g., Dachshunds, French Bulldogs). Symptoms: Diagnosis and Treatment: Conclusion Orthopedic conditions in veterinary medicine can significantly impact an animal’s quality of life. Early diagnosis and appropriate intervention are crucial to successful management. Advances in diagnostic imaging, surgical techniques, and biomaterials continue to improve outcomes in veterinary orthopedics. By understanding these common orthopedic issues, veterinarians can offer more effective, personalized treatment plans to enhance mobility and overall well-being in their patients. #VeterinaryOrthopedics #AnimalHealth #Boneadhesive #FractureRepair #JointCare #Osteoarthritis #VeterinarySurgery
Release Of Our Very First OssiVet® Order!
The PBC Biovet Operations Team has reached a significant milestone with the preparation and release of our very first OssiVet® order! OssiVet®, our innovative veterinary bioadhesive bone substitute, is designed to support advanced orthopedic care for companion animals, combining safety, efficacy, and ease of use. This landmark achievement is a testament to our team’s hard work, dedication, and commitment to delivering innovative solutions for veterinary orthopedic Application/ Fixation. 🔹 State-of-the-art preparation: Our team ensures the highest quality standards throughout the development lifecycle. 🔹 Seamless execution: From production to distribution, every step reflects our passion for excellence. 🔹 Onward to veterinarians: OssiVet® is now on its way to revolutionize bone surgeries in veterinary practices. We are proud to contribute to advancing veterinary care and look forward to hearing success stories from veterinarians using OssiVet® in their practices. #VeterinaryInnovation #PBCBiovet #OssiVet #Orthopedicsurgery

Accelerating new Medical Innovations: OTA Showcase
PBC Biomed are ready for action at the Orthopaedic Trauma Association meeting in Montreal! Stop by booth #431 to meet our team and learn more about our new #innovations, powered by our Accelerating Innovation Model ‘AIM’. #acceleratingmedicalinnovation #biomaterials #newinnovations
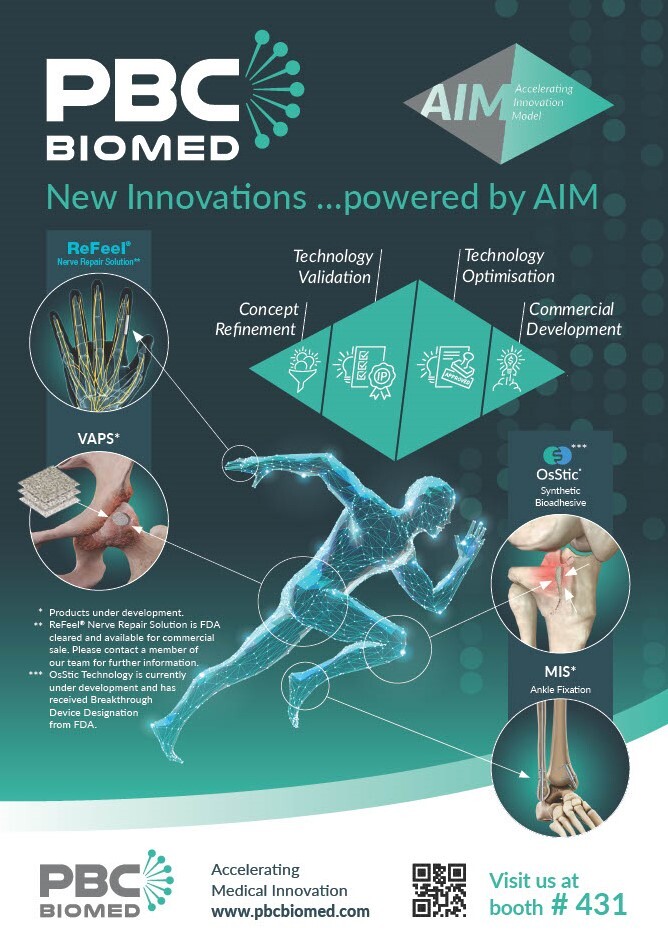
PBC Biomed OTA Symposium – Strengthening the Bond: Modern Biomaterials for Fracture Fixation and Bone Regeneration.
We are delighted to announce that we will be continuing our ‘Strengthening the Bond’ education series at the Orthopaedic Trauma Association meeting in Montreal this week. Strengthening the Bond: Modern Biomaterials for Fracture Fixation and Bone Regeneration. Our global panel of surgeon advisors will discuss current hashtag#Biomaterials and their challenges, as well as plans for the first human implantation of OsStic®, a hashtag#Breakthrough Bio-Adhesive bone void filler technology. Speaker panel is as below: – Peter Giannoudis – John Tracy Watson – Hassan Mir, MD, MBA – Thomas A. (Toney) Russell MD The session will take place on Friday 23rd October at 12:45pm in room 514ABC. See below for further information – we look forward to seeing you there! #OTA2024 #strengtheningthebond #industrylunchseries #pbcbiomed #acceleratingmedicalinnovation #biomimeticinnovations

DTIF Project Consortium Progress Meeting
It was fantastic to welcome the Enterprise Ireland #DTIF consortium team to our Shannon Headquarters at the start of September for a project progress meeting. Thank you to Nicholas Dunne and the DCU postgraduate team, the Dolmen Design & Innovation team, as well as Jackie FitzGerald of Enterprise Ireland for making the trip! #DTIF #disruptivetechnology #OsStic #development #researchanddevelopment #enterpriseireland #acceleratingmedicalinnovation

PBC Biomed supports Day One Trauma Charity’s House of Lords Event
The PBC Biomed team had the pleasure of joining the volunteers, supporters and sponsors of Day One Trauma Support Charity for a reception at the House of Lords in London last week. PBC Biomed are a sponsor of this fantastic charity and their Major Trauma Awareness Week campaign. Check out their page for details on their inspiring#DearMe project, which shares powerful stories from people who have experienced life-changing injuries. Pictured are Patrick Rickard Financial Controller and Paul Burke, Managing Partner at PBC Biomed; Professor Peter Giannoudis, Founder of Day One Trauma Charity; Gerard Insley, Chief Science Officer at PBC Biomed; Lucy Nickson, CEO of Day One Trauma Charity; and Melissa Ferreira, Executive Assistant at PBC Biomed. The team presented Professor Giannoudis with the European Patent Plaque, granted for the Vascularity Affinity Precursor Scaffold. This technology is an autologous cell delivery scaffold designed to treat and repair necrotic or dead bone defects. PBC Biomed are currently developing this scaffold for the treatment of avascular necrosis patients. More to come on this shortly! #MajorTraumaAwarenessWeek2024 #DayOneTrauma #PBCBiomed #Acceleratingmedicalinnovation

ESTROT 2024
PBC Biomed are delighted to sponsor and confirm attendance at the 8th Annual European Society of Tissue Regeneration in Orthopaedics and Traumatology (ESTROT) meeting in Helsinki, Finland next week. This year’s event will focus on: – Biomaterials: tissue regeneration, infection & treatment– Bone & Cartilage: tissue reconstruction & regeneration– Traumatology: surgical techniques, host reactions– Plastic Surgery: soft tissue regeneration– Tissue Regeneration in Children We look forward to three days of stimulating discussion and learning, as well as an opportunity to catch up with European thought-leaders. Nähdään siellä! #ESTROT #trauma #regenerativemedicine #pbcbiomed #orthopaedics #trauma

DTIF-funded project, OsStic® technology awarded #BreakthroughDevice designation by the FDA.
Shannon, Ireland, February 12th 2024: Irish medical device company Biomimetic Innovations Ltd, an affiliate of PBC Biomed, announced ‘Breakthrough Device’ designation from the Food and Drug Administration FDA in January, for the development of a groundbreaking new bio-adhesive bone void filler technology called OsStic®. PBC Biomed leads a consortium for this development project, which is comprised of Biodesign Europe and SFI Research Centre for Advanced Manufacturing (I-Form) at Dublin City University, and Dolmen Design & Innovation, a Dublin-based product design company. The project (2021 – 2024) is funded under the Disruptive Technologies Innovation Fund (DTIF), which is a €500 million fund established under the National Development Plan (NDP) in 2018 and led by the Department of Enterprise, Trade and Employment, and administered by Enterprise Ireland. The aim of the fund is to drive collaboration between Ireland’s world-class research base and industry, as well as facilitating enterprises to compete directly for funding in support of the development and adoption of these technologies. Welcoming the announcement, Dr Imelda Lambkin, Disruptive Technologies and Innovation Department Manager, Enterprise Ireland commented; “This announcement is a great success for PBC Biomed and is testament to the capability and talent, vibrancy and international competitiveness of the Irish innovation and research system. In line with Enterprise Ireland’s strategy, this success will support the company to build on their existing capabilities, scale and create jobs. “The collaboration between Dublin City University, Dolmen Design & Innovation, Biomimetic Innovations, and PBC Biomed exemplifies the power of interdisciplinary efforts in research and development. The ‘Breakthrough Device’ designation by the FDA validates the promising trajectory of OsStic®, showcasing Ireland’s capability to lead in cutting-edge medical technologies. This achievement not only highlights the success of the project but also emphasizes the importance of sustained investment in disruptive technologies to foster innovation and elevate Ireland’s global standing in the medical device industry.” Professor Nicholas Dunne, Founding Executive Director of Biodesign Europe, Dublin City University and Funded Investigator in the SFI Research Centre for Advanced Manufacturing and SFI Centre for Advanced Materials and BioEngineering Research. “This announcement marks a major milestone in the evolution of calcium-based biomaterials. The proposed indication will be unique and for the first time will address all surgeon needs when dealing with complex fractures and allow for faster recovery times and greatly improved results for patients.” Gerard Insley, Chief Science Officer BMI. Learn more about DTIF here: https://lnkd.in/dHQ3_2vF About BMI Biomimetic Innovations Ltd, is an affiliate of PBC Biomed; a medical device company involved in design, development and manufacturing headquartered in Shannon, Ireland and with offices in Memphis, Tennessee and Chamonix, France.
FDA grants global Medical Device company Biomimetic Innovations ‘Breakthrough Device’ designation for OsStic® Synthetic Injectable Structural Bio-Adhesive Bone Void Filler.
Shannon, Ireland, 19th January, 2024: Biomimetic Innovations Ltd today announced that OsStic® has been granted ‘Breakthrough Device’ designation by the FDA. The proposed indication statement for this novel new technology is; ” OsStic®Synthetic Injectable Bone Void Filler is a structural, mechanically enhanced bioadhesive for reduction, provisional fixation, or void filling of peri-articular fractures or defects to enhance structural stability where standard fixation alone cannot provide sufficient support for functional mobilization.” This Breakthrough Designation of our disruptive technology fuels our passion for earlier mobilization of trauma patients. Paul Burke, Director BMI. The Breakthrough Devices Program is a voluntary program for certain medical devices and device-led combination products that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. [source] The program is intended to provide patients and health care providers with timely access to medical devices, by speeding up development, assessment, and review for premarket approval, 510(k) clearance, and De Novo marketing authorization. [source] OsStic® pioneers the evolution of structural orthobiologics, to the point where surgeons can now use this material as an aid for the reduction, provisional fixation and void filling of peri-articular fractures. This is the first calcium phosphate that meets all these clinical requirements. Dr Thomas A Russell, CMO BMI. About BMI Biomimetic Innovations Ltd, is an affiliate of PBC Biomed; a medical device company involved in design, development and manufacturing headquartered in Shannon, Ireland and with offices in Memphis, Tennessee and Chamonix, France. Contact Details Bronagh O’Doherty, Global Product Manager, PBC Biomed. Email: [email protected] Reference (Image) Lundin TPO, Pujari-Palmer M, Svensson G, Höglund OV. Canine ex vivo tarsal arthrodesis: fixation by using a new bone tissue glue. Front Vet Sci. 2023 Sep 20;10:1250147. doi: 10.3389/fvets.2023.1250147. PMID: 37799403; PMCID: PMC10548131. Link: https://www.frontiersin.org/articles/10.3389/fvets.2023.1250147/full

Prof. Luke O’Neill showcasing our partner ZOAN Biomed
Zoan G03, a product created by our partner Zoan Biomed was showcased yesterday evening on new RTÉ show ‘Future Island’. ZoanG03 is a bioactive bone healing device. The corals’ Natural Assembly Process ensures every surface, every pore and every mineral contribute to building a living scaffold. Superior bioactivity stimulates speedy and complete acceptance by the patient leading to accelerated rates of healing. Manufacture of ZoanG03, a bioactive granule for bone healing and repair commences Q1 2021, supported by the EU EMMF, Coral4Health project. The full show can be watched on the RTÉ player at this link.

