Kicking Off a New Partnership with Colorado State University’s Translational Medicine Institute
Kicking Off a New Partnership with Colorado State University’s Translational Medicine Institute Chief Medical Officer Dr Russell, R&D Director Paul Dorrell, R&D Engineer Antzela Tzagiollari are just back from a successful trip to the C. Wayne Mcilwraith Translational Medicine Institute in Colorado. Colorado State University’s Translational Medicine Institute is a state-of-the-art research hub which brings scholars, creators, and entrepreneurs together to work in a collaborative space where innovation thrives. The Institute’s goal is to discover and deliver solutions that utilize the body’s healing capacity and improve the lives of animals and the humans who care for them. This goal aligns perfectly with our mission to enhance patient well-being through the development of innovative new medical devices. We are delighted to be kicking off our partnership with this group! #biomimeticinnovations #acceleratingmedicalinnovation #researchanddevelopment

OssiVet® Now Available in EU
OssiVet® Now Available in EU We’re excited to announce that OssiVet®, our innovative Synthetic Adhesive Bone Substitute, is now available for use in domestic companion animals for orthopedic reduction, provisional fixation, and void filling of bone fractures or defects. OssiVet® is designed to make a difference in your practice by offering: • Simplified application for improved surgical outcomes. • Biomimetic Processes: Ossivet® bone substitutes are designed to mimic the natural adhesion, mechanical properties, and remodelling of bone, facilitating bone healing, defect repair, and implant fixation. • Supporting a variety of animal orthopaedic cases with confidence. 🎯 Interested in seeing OssiVet® in action? We’re offering exclusive product demonstrations to show how it can transform your practice. 👉 reach out to schedule your demo or to learn more about OssiVet®. [email protected] Let’s work together to enhance small animal orthopaedic care Stay tuned for more country availability and follow us. www.pbcbiomed.com/pbcbiovet #ossivet #PBCBiovet #VeterinaryInnovation #AnimalCare #animalorthopedics #EULaunch

Innovation Lab at University of Leeds
Innovation Lab at University of Leeds Last week the PBC Biomed team held their first innovation lab of the year in the University of Leeds Teaching Hospital. Our Clinical Advisory Board – Dr Hassan Mir, MD, MBA, Dr John Tracy Watson and Dr Thomas A. (Toney) Russell MD and our host Professor Peter Giannoudis, were joined in the lab by US Partners Sanara MedTech, as well as our Japanese Colleagues from Mochida Pharmaceutical Ltd. We were delighted to welcome this group to Leeds to explore our #innovative technologies – our passion for ‘enhancing patient wellness’ remains central to all our development programmes! We were also delighted to have Kirsty Christmas and the Day One Trauma Support team stop by. Kirsty kindly updated the group on the activities of the charity (founded by Professor Giannoudis in 2014), the fantastic work that they continue to do for patients requiring support following major trauma, as well as it’s growing success over the last ten years. What a team!

Biomimetic Innovations Ltd Announces Exclusive License and Distribution Agreement with Sanara MedTech Inc
We are proud to announce that our affiliate partner Biomimetic Innovations Limited(BMI), has announced the execution of an exclusive license and distribution agreement with Sanara MedTech Inc. Sanara agreed to invest in BMI with cash for equity at the same time. Sanara, a medical technology company based in Fort Worth, Texas, with attention to developing and commercializing cutting-edge technologies to improve clinical outcomes and reduce expenses for healthcare in the chronic wound, surgical and skincare markets. Sanara now has the exclusive U.S. marketing, sales, and distribution rights to OsStic® Synthetic Injectable Structural Bio-Adhesive Bone Void Filler (“OsStic®”) under the terms of the License and Distribution Agreement, as well as a hardware adaptable supplementary internal fixation technology featuring a newly to promote targeted application of OsStic®, for use in fracture management. The License and Distribution Agreement is for an initial five-year period, with the option to automatically renew for another two years at the discretion of Sanara. Developed by Biomimetic Innovations Ltd, OsStic®, is a disruptive new bio-adhesive bone void filler technology which was granted a Breakthrough Device Designation by the U.S. Food and Drug Administration (“FDA”) on December 10, 2023. The proposed indication statement for OsStic® is [a] “structural, mechanically enhanced bioadhesive for reduction, provisional fixation, or void filling of periarticular fractures or defects to enhance structural stability where standard fixation alone cannot provide sufficient support for functional mobilization.” Breakthrough Device Designation is granted to medical devices that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating human diseases or conditions. “We are excited to announce this strategic agreement and investment in Biomimetic Innovations,” said Ron Nixon, Sanara’s Executive Chairman and CEO. “OsStic® offers a truly differentiated solution, designed to enhance the fracture repair process. We believe OsStic® represents a compelling treatment option for the more than 100,000 periarticular fractures that occur in the U.S. annually.” Dr. Thomas Russell, Chief Medical Officer of PBC Biomed (an affiliate of BMI), stated, “Unlike conventional materials, OsStic® is engineered to optimize fluidic dispersion into bone defects, interdigitate with the surrounding boney structure, and firmly adhere to bone surfaces. These properties provide exceptional structural integrity and mechanically enhanced bioadhesion, making OsStic® uniquely suited for reducing periarticular fractures, achieving provisional fixation, and filling voids. As the first calcium phosphate-based synthetic technology to address all three critical clinical needs, OsStic® offers an unparalleled solution for preserving joint congruency and improving patient outcomes.” Managing Partner of PBC Biomed Ltd and the acting CEO of Biomimetic Innovations, Paul Burke, stated, “Our goal is to ‘enhance patient wellness’ using disruptive medical innovations. With its market expertise, sales and distribution network, and existing customer relationships, Sanara represents an ideal partner to commercialize our innovative technologies. Together, we look forward to bringing transformative solutions to market that enhance the surgeon’s treatment approach and improve patient outcomes.” #bioadhesive #Bio-Adhesive #biomaterials #biomimetcinnovations #breakthroughDesignation #medtech #partnership #sanara #innovation

Happy New Year from all of us at PBC Biomed Group!
Our mission of ‘Enhancing Patient Wellness’ continues to drive us and will be central to all our activities in 2025. Providing clinical solutions in both Human and Veterinary through our newly released products, *ReFeel® Nerve Repair Solution, HANIA Skin Protectant and OssiVet (Bioadhesive) will be key focus areas, while we continue to work with our academic and industrial partners on new product developments. Join us Accelerating Medical Innovation! #pbcbiomed #acceleratingmedicalinnovation #newyearnewopportunities

New Product Development Lab – MERI, October 2024.
Our passion for innovation continues with our New Product Development Lab in Memphis this week – the photos of the team speak for themselves! Potential indications, design inputs and procedural techniques for our new technology pipeline drove their agenda for the two days. Thank you to the team at Medical Education & Research Institute for making this lab a success and supporting our mission to enhance patient well-being. #acceleratingmedicalinnovation #PBCBiomed #biomaterials #orthopaedic #biomaterials #researchanddevelopment #productpipeline

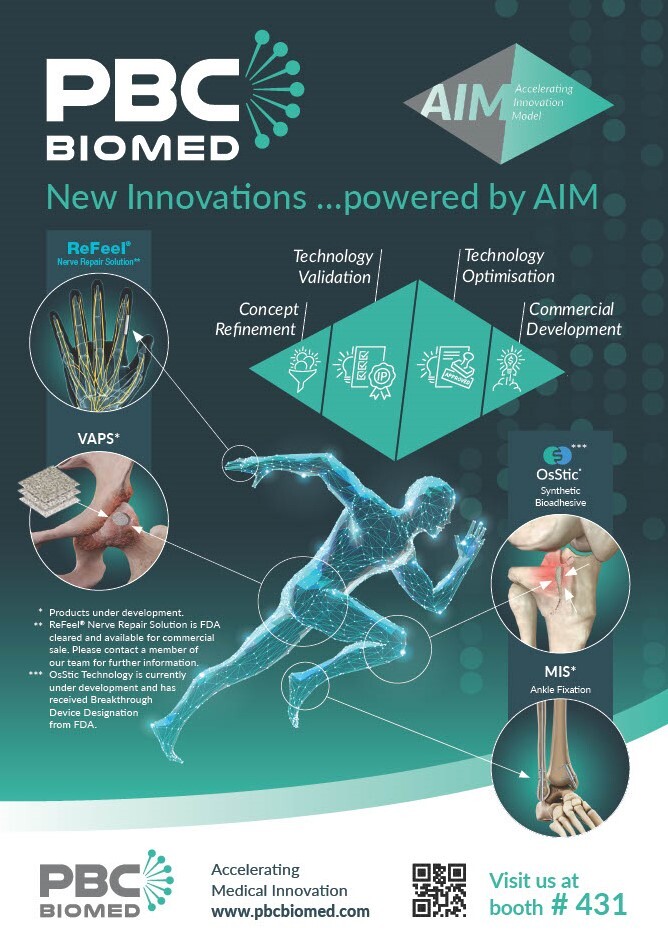
Accelerating new Medical Innovations: OTA Showcase
PBC Biomed are ready for action at the Orthopaedic Trauma Association meeting in Montreal! Stop by booth #431 to meet our team and learn more about our new #innovations, powered by our Accelerating Innovation Model ‘AIM’. #acceleratingmedicalinnovation #biomaterials #newinnovations
PBC Welcomes Mochida to Memphis
PBC Biomed Inc welcomed the Mochida team to their Epicenter office in Memphis last week, ahead of the ReFeel Nerve Repair Solution launch.

New Milestone for PBC Biomed & client Mochida Pharmaceutical., Ltd.
In September, PBC Biomed and our client Mochida Pharmaceutical., Ltd. hit a significant milestone in our co-development journey. Together, we launched ReFeel® at the American Society for Surgery of the Hand (ASSH) meeting event in Minneapolis, where we demonstrated the technology publicly for the first time with the help of our surgeon advisors. Thank you to Dr Rashard Dacus, Dr Glenn Gaston and Dr William Jake Weller for stopping by our booth to demonstrate application of ReFeel® using our new training models, as well as their guidance throughout the development process of this exciting new technology. We are thrilled to release this new product in the US for treatment of patients with peripherical nerve discontinuities and management of nerve injuries. #ReFeel® #nerverepairsolution #acceleraratingmedicalinnovation #ASSH24

DTIF Project Consortium Progress Meeting
It was fantastic to welcome the Enterprise Ireland #DTIF consortium team to our Shannon Headquarters at the start of September for a project progress meeting. Thank you to Nicholas Dunne and the DCU postgraduate team, the Dolmen Design & Innovation team, as well as Jackie FitzGerald of Enterprise Ireland for making the trip! #DTIF #disruptivetechnology #OsStic #development #researchanddevelopment #enterpriseireland #acceleratingmedicalinnovation

