Biomimetic Innovations Ltd Announces Exclusive License and Distribution Agreement with Sanara MedTech Inc
We are proud to announce that our affiliate partner Biomimetic Innovations Limited(BMI), has announced the execution of an exclusive license and distribution agreement with Sanara MedTech Inc. Sanara agreed to invest in BMI with cash for equity at the same time. Sanara, a medical technology company based in Fort Worth, Texas, with attention to developing and commercializing cutting-edge technologies to improve clinical outcomes and reduce expenses for healthcare in the chronic wound, surgical and skincare markets. Sanara now has the exclusive U.S. marketing, sales, and distribution rights to OsStic® Synthetic Injectable Structural Bio-Adhesive Bone Void Filler (“OsStic®”) under the terms of the License and Distribution Agreement, as well as a hardware adaptable supplementary internal fixation technology featuring a newly to promote targeted application of OsStic®, for use in fracture management. The License and Distribution Agreement is for an initial five-year period, with the option to automatically renew for another two years at the discretion of Sanara. Developed by Biomimetic Innovations Ltd, OsStic®, is a disruptive new bio-adhesive bone void filler technology which was granted a Breakthrough Device Designation by the U.S. Food and Drug Administration (“FDA”) on December 10, 2023. The proposed indication statement for OsStic® is [a] “structural, mechanically enhanced bioadhesive for reduction, provisional fixation, or void filling of periarticular fractures or defects to enhance structural stability where standard fixation alone cannot provide sufficient support for functional mobilization.” Breakthrough Device Designation is granted to medical devices that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating human diseases or conditions. “We are excited to announce this strategic agreement and investment in Biomimetic Innovations,” said Ron Nixon, Sanara’s Executive Chairman and CEO. “OsStic® offers a truly differentiated solution, designed to enhance the fracture repair process. We believe OsStic® represents a compelling treatment option for the more than 100,000 periarticular fractures that occur in the U.S. annually.” Dr. Thomas Russell, Chief Medical Officer of PBC Biomed (an affiliate of BMI), stated, “Unlike conventional materials, OsStic® is engineered to optimize fluidic dispersion into bone defects, interdigitate with the surrounding boney structure, and firmly adhere to bone surfaces. These properties provide exceptional structural integrity and mechanically enhanced bioadhesion, making OsStic® uniquely suited for reducing periarticular fractures, achieving provisional fixation, and filling voids. As the first calcium phosphate-based synthetic technology to address all three critical clinical needs, OsStic® offers an unparalleled solution for preserving joint congruency and improving patient outcomes.” Managing Partner of PBC Biomed Ltd and the acting CEO of Biomimetic Innovations, Paul Burke, stated, “Our goal is to ‘enhance patient wellness’ using disruptive medical innovations. With its market expertise, sales and distribution network, and existing customer relationships, Sanara represents an ideal partner to commercialize our innovative technologies. Together, we look forward to bringing transformative solutions to market that enhance the surgeon’s treatment approach and improve patient outcomes.” #bioadhesive #Bio-Adhesive #biomaterials #biomimetcinnovations #breakthroughDesignation #medtech #partnership #sanara #innovation

Alginate Use in Orthopedics and Peripheral Nerve Repair
We are excited to announce the release of new publication: ‘Alginate Use in Orthopedics and Peripheral Nerve Repair: A Systematic Review’; which is now live on the Cureus website. The aim of the paper is to examine alginate’s use in orthopaedic indications, as well as look at future applications of alginate for peripheral nerve repair. This publication was written by Dr Joshua Hustedt who based out of Banner Health, in Phoenix AZ. Dr. Joshua Hustedt, MD/MHS is a double board-certified, fellowship trained, hand, upper extremity, and peripheral nerve surgeon. Dr Hustedt has worked as part of the ReFeel development team with our partner company and legal manufacturer Mochida Pharmaceutical Ltd. Full publication can be accessed below – congratulations to Dr Hustedt on its release! https://www.cureus.com/articles/302391-alginate-use-in-orthopedics-and-peripheral-nerve-repair-a-systematic-review #ReFeelnerverepairsolution #peripheralnerverepair #nerverepair #alginate #orthopaedics #acceleratingmedicalinnovation

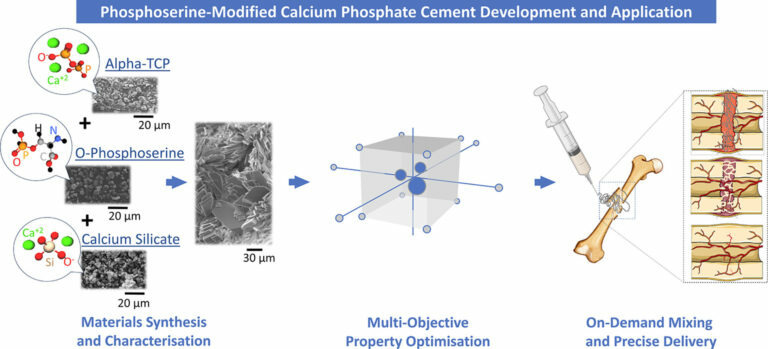
New Publication: ‘Multi-objective property optimisation of a phosphoserine-modified calcium phosphate cement for orthopaedic and dental applications using design of experiments methodology.’
Biomimetic Innovations Ltd R&D Engineer Antzela Tzagiollari and the research team at DCU recently published a paper titled; ‘Multi-objective property optimisation of a phosphoserine-modified calcium phosphate cement for orthopaedic and dental applications using design of experiments methodology.’ This publication explains how their DoE approach successfully optimised the composition of OsStic®, demonstrating that the liquid: powder ratio (LPR) and quantity of phosphoserine (wt%) significantly influences the material’s handling, mechanical and adhesion properties. Subsequently, the DoE optimisation process identified the optimal formulation for OsStic®; demonstrating rapid setting, achieving complete solidification within 5 minutes, while also achieving high mechanical and adhesive properties (compressive strength of 29.2 ± 4.9 MPa and bond/shear strength of 3.6 ± 0.9 MPa). Additional findings highlighted the ability of OsStic® to effectively support and stabilise bone fragments during the initial stages of natural bone healing; showing that this ground-breaking new material fulfils the clinical requirements for working and setting times, static mechanical, degradation properties, and injectability. This further supports our belief that OsStic will enable surgeons to stabilise complex bone fractures; representing a significant advancement in treatment options, as well as the potential to greatly enhance patient outcomes. Read the full publication here.
PBC Biomed Clinical Advisory Board publish Editorial in Injury Journal.
Part One of our ‘Strengthening the Bond’ symposium series at the Orthopaedic Trauma Association meeting in Tampa, focused on the unanswered challenges of osteosynthesis, as well as a first look at research on a promising new bio-adhesive. This surgeon panel, consisting of Professor Peter Giannoudis, Dr Tracy Watson, Dr Thomas A Russell, Dr Hassan Mir and Dr Alicja Bojan, have now subsequently published an editorial summarising this discussion. Editorial| Volume 54, Issue 12, 111154, December 2023. Modern osteosynthesis of periarticular fractures: The role of provisional fixation revisited. Available to view here: https://www.injuryjournal.com/article/S0020-1383(23)00871-9/abstract #acceleratingmedicalinnovation #Periarticular #Provisional fixation #Osteosynthesis

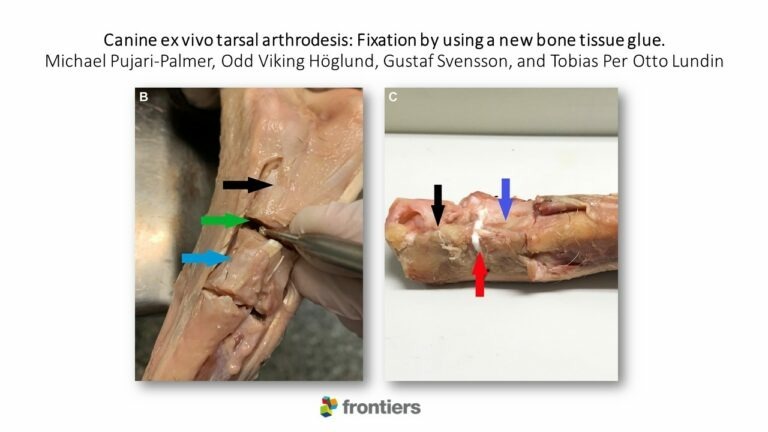
Canine ex vivo tarsal arthrodesis: Fixation by using a new bone tissue glue.
Researchers from the Department of Clinical Sciences, Swedish University of Agricultural Sciences, Uppsala, Sweden (SLU) and the Department of Surgery, Blå Stjärnans Djursjukhus, Gothenburg, Sweden; Michael Pujari-Palmer, Odd Viking Höglund, Gustaf Svensson, and Tobias Per Otto Lundin, recently published a paper which examines the fixation capabilities of OsSticTM technology in a canine ex vivo tarsal arthrodesis test model. Arthrodesis of the tarsal joint has a high incidence of postoperative complications and intraoperative difficulties. An adhesive that facilitates implant placement and joint alignment, as well as strengthening the internal fixation, could potentially be a useful complementary clinical tool to reduce the risk of complications in these high-risk procedures. In this experimental ex vivo study, a new bone glue – OsSticTM technology, was evaluated as a subchondral bone adhesive. This glue is composed of materials that are present in the body; calcium and silicate, and the amino acid phosphoserine. The biomechanical model the research group developed was sensitive enough to measure differences in fixation strengths between different glue formulations. The average fixation strength was 60–100 N/cm2, which should be strong enough to support short-term load bearing in medium sized canines (20 kg). This demonstrates that this new resorbable glue – OsSticTM, can potentially contribute to stability at arthrodesis surgery, acting as a complement to today’s standard fixation, metal implants. Read the full publication here.
‘Octacalcium Phosphate Biomaterials’ is now available
PBC Biomed is delighted to announce that a new book, ‘Octacalcium Phosphate Biomaterials’ has recently been published and is available for sale. The book is a comprehensive study of octacalcium phosphate (OCP), a next generation biomaterial for bone regeneration, including its potential for clinical application KEY FEATURES • Contains comprehensive, up-to-date information on the basic science, including physical, chemical and biological properties • Presents the clinical potential of octacalcium phosphate biomaterials • Provides a reference point for new research and increased activity in the area of next generation smarter biomaterials for hard tissue repair and regeneration DESCRIPTION Octacalcium Phosphate Biomaterials: Past, Present and Future is a comprehensive study of octacalcium phosphate (OCP), a next generation biomaterial for bone regeneration. By focusing both on fundamental research and the use of OCP as a scaffold material, this book explores its potential to deliver improved clinical results. OCP is known to be a pre-cursor to hydroxyapatite in the human biomineralization process that forms bone and tooth enamel. Research studies that have emerged in recent years suggest OCP’s tremendous potential as a bioactive material. Gerard Insley PhD is Chief Scientific Officer with PBC Biomed. This book forms part of our company’s strategy of Developing new Osteostimulative Biomaterials for Compromised bone Regeneration.

Octacalcium Phosphate Biomaterials
Octacalcium Phosphate shows increased Alkaline Phosphatase activity versus Calcium Phosphates. Read more in the shortly to be published book. For more information contact [email protected]

